In many pH applications, the fragile glass electrode needs to be replaced by a nonglass pH electrode alternative. For example, in food processing a glass breakage can bring the whole process to stop, no matter how small glass fragments.
The pH glass electrode technology has been available for a long time, and even it appears there have been few new developments in the nonglass alternative on the market for more than two decades.
Basic function
For a glass electrode, it is the probe tip, the glass membrane, that is the hydrogen sensitive part. For the nonglass pH electrode, the pH sensitive glass has been replaced by a hydrogen ion-selective field effect transistor (ISFET).
The behavior of both ISFET and glass electrodes can be described by the Nernst equation. The sensitivity of the ISFET electrode is comparable with that of a glass pH electrode. However, the zero point of the ISFET electrode is different from that of most glass electrodes. Which means pH 7 is not at zero mV but can be 100 mV or more. For more about sensitivity (slope) and zero point, see the calibration page.
Principle of operation of the
nonglass pH electrode
The ISFET uses a different mechanism for measuring pH from that of traditional glass electrodes. The measurement principle is based on the control of the current flowing between two semiconductor electrodes. These two electrodes, the so-called drain and source electrodes, are placed in a silicon chip together with a third electrode, the so-called gate between them. The gate is in direct contact with the solution to be measured.
The pH sensitive part
The gate electrode consists of a special chemical layer, which is sensitive to hydrogen ions. Materials like silicon oxide (SiO2), silicon nitride (Si3N4) and aluminium oxide are used in the pH sensing layer.
Hydrogen ions will reside at the surface of the chemical layer in proportion to the pH. The positive charge of the hydrogen ions produces an electric field that influences the current between the source and drain. So when the pH value changes, the current through the transistor will change accordingly. To maintain the drain–source current at a constant value a control voltage has to be applied via the reference electrode.
The change in the control voltage (UGS) is a measure of the pH value of the sample.
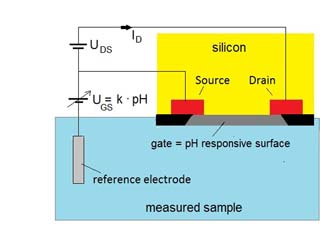
Reference electrode
The reference electrode works the same as for a conventional glass electrode. This means that for an ISFET electrode exist the same problems and choices with the reference electrode as for the glass electrode.
Design of an ISFET electrodeisfet electrode
The ISFET-chip is sealed in a hermetic enclosure and mounted at the tip of the sensor body. As the ISFET electrode is a nonglass pH electrode, the electrode body is made of a plastic (epoxy or polymer). The plastic body is a good choice for applications where a glass breakage is unacceptable.
An ISFET electrode can easily be made very small, as an ISFET chip is a lot smaller than a glass membrane. However, for normal use in a laboratory or process environment the size of ISFET electrodes matches the size of glass electrodes.
Temperature
As with the glass electrode, most ISFET electrodes have a temperature sensor embedded in the body.
Nonglass pH meter
As already mentioned on the pH meter page, a glass electrode has a very high internal resistance and to make reliable measurements the input resistance of the pH meter has to be even higher. However, this differs from an ISFET nonglass pH electrode. An ISFET electrode combines in one device, the pH sensing transistor and a signal amplifier which produces a high current, low impedance output. This means you have to use a pH meter with an interface adapted to an ISFET electrode.
Some of the ADVANTAGES with an ISFET electrode compared to a traditional glass electrode.
ISFET electrodes:
+ are virtually unbreakable and there is no risk of broken glass.
+ are very rugged and the pH sensing area can be easily cleaned with a toothbrush.
+ can be stored dry. pH glass electrodes must be stored in an aqueous environment to prevent dehydration.
+ have a short response time and give fast measurements.
+ can reduce the acidic or alkaline errors in extreme pH conditions without the need to resort to a special glass.
Some of the DISADVANTAGES with an ISFET electrode compared to a traditional glass electrode.
ISFET electrodes:
- are more expensive than pH glass electrodes.
- do not offer the same stability and accuracy afforded by glass electrodes.
- have a known drifting problem.
- are highly temperature dependent and there is a risk of damage by rapid cycling temperature changes.
- are sensitivity to direct sunlight. Like all semi-conductor elements, the ISFET chip is sensitive to light (measured value fluctuations).
- will only work with pH meters, which are adapted to the ISFET technology and are not interchangeable with meters for conventional pH glass electrodes.
- should not be used in conjunction with chlorine or other chemicals which will permanently damage the ISFET chip.
Note:
The ISFET electrode characteristics can differ between manufactures and new development can reduce earlier disadvantages.
For information on your specific nonglass pH electrode, see the instruction manual that came with your electrode.
Compiled by Rami E. Kremesti M.Sc.