GMP Quality Ultra Pure Water for Pharmaceutical Industry
The water used in the pharmaceutical industry, used both in drug synthesis, cleaning, as well as Water for Injection needs to meet strict quality requirements according to various Pharmacopeia. Water Treatment Systems and process equipment for the pharmaceutical industry need to be sanitizeable by steam and for this reason materials are used that can withstand high temperatures. Highly polished materials (mostly SS) need to be used to prevent the growth of biofilm. Sanitary pumps are used that minimize particle shedding and no ball valves are allowed because they can harbor biofilm.
The eight types of water used in the pharmaceutical industry are:
1. Non-potable for cooling
2. Potable (drinkable) water
3. USP purified water
4. USP water for injection (WFI) - Distillation must be used for this type of water according to European Pharmacopeia
5. USP sterile water for injection
6. USP sterile water for inhalation
7. USP bacteriostatic water for injection (contains an anti-bacterial agent)
8. USP sterile water for irrigation - irrigation in medicine means the washing of a body cavity or wound by a stream of water
Pure water for various pharma applcations needs to be demineralized, the organics and SS in it removed and the water disinfected/sterilized to make sure there are no bacteria. Technologies such sa Micro-Filtration, UF, RO, IEX, EDI, Distillation and UV are used. Stored water is nornally circulated at a high temperature to prevent biofilm growth. Systems are designed to prevent dead legs and allow easy drainage for cleaning. The facilities housing the water treatment equipment must be controlled and prevent cross contamination from outside.
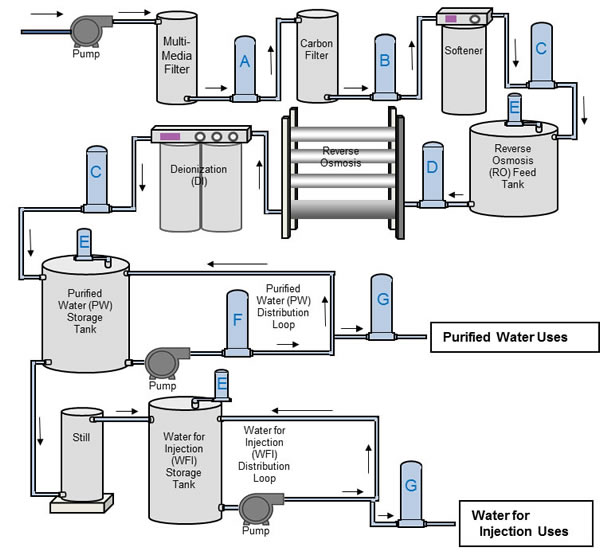
A- Particle Trap Filtration
B- Carbon Fines Trap
C- Resin Trap
D- RO Prefilter
E- Tank Vent Filter
F- Bioburden Reduction
G- Sterilizing Filter
Quality parameters for Pharma water are: conductivity, various ions, particles (SS), bacteria and endotoxins. Absence of bacteria is a must for higher quality waters.
Some of the standards of reference for UPW for Pharma are:
1. FDA Guide to Inspections of High Purity Water Systems, High Purity Water Systems (7/93)
2. USP Monograph <1231> Water For Pharmaceutical Purposes
3. WHO good manufacturing practices: water for pharmaceutical use
When an UPW system for pharma is installed and commissioned, it must undergo Installation qualification (IQ), Operational qualification (OQ) and Performance qualification (PQ).
Ultra Pure Water systems are validated and qualified to make sure that the water they produce is consistent with the GMP requirements. For example, periodic tests for leachables are performed to make sure that the equipment producing the UPW is not leaching TOC. Periodic tests for bacteria and pyrogens/endo toxins are also made.
Waste water generated by the pharmaceutical industry also needs to be treated to meet strict discharge limits and Good Environmetnal Practice. High COD waters and active pharmaceutical ingredients need to me removed. In fact there is published data and investigations that assert that pharmaceuticals are making it into the environment either through waste water discharge from pharmaceutical plants, through human sanitary waste and through lanfill waste leachate. Read this article for more info. Biological treatment is one method that is used, but if the COD is very high, incineration or Wet Air Oxidation might be needed.
The activated sludge process does not lend itself very well to pharmaceutical waste water treatmant, the reason being that API's are generally not biodegradable. RBC waste water treatement plants have shown better results. MBR treatment can concetrate the API's too if the molecules are large and cannot pass through the MBR membrane and hence reduce the amount of waste water. UV/H2O2 treatment has also shown good results and is in use for CIP waste water treatment. Note that two wavelengths of UV light are used, 254 and 185 nm. The higher wavelength water kills bacteria, the lower one is so strong it even generates Ozone which breaks down organics.
Another very important concept in the pharmaceutical industry is GMP and Validation.
Validation can be defined as process of establishing through documented evidence a high degree of assurance that a specific process will consistently produce a product that meets its predetermined specifications and quality attributes. A validated manufacturing process is one that has been proven to do what it purports or is represented to do. The proof of validation is obtained through collection and evaluation of data, preferably beginning from the process development phase and continuing through the production phase. Validation necessarily includes process qualification (the qualification of materials, equipment, systems, buildings, and personnel), but it also includes th
1. Cleaning Validation
Cleaning validation is carried out to ascertain the procedure and method adapted for cleaning of equipments , and areas , is capable of giving desired cleanness , cleanliness of equipment can be ascertained by caring out trace analysis of active ingredient of previous products active ingredient trace analysis . By doing rinse water TOC analysis or swab tests, the complete removal of earlier products residue is ascertained so as to avoid cross contamination. Bioburden is defined as the number of bacteria living on a surface that has not been sterilized. The term is most often used in the context of bioburden testing, also known as microbial limit testing, which is performed on surfaces for quality control purposes.
2. Process Validation
Process validation is carried out on the manufacturing process or steps , which are adapted for pharmaceutical manufacturing . The process adapted in pharma manufacturing should yield consistent results with respect to quality of product. The laid down process is crosschecked for evidence for efficacy, and the results are documented for each step.
3. Method Validation
In Pharma QC labs, only validated methods/qualified instruments are used to
analyse for various API's and only trained personnel are allowed to runs these methods.
FDA guidelines define process validation as follows:
Cleaning Validation proves that the washing of the reactor for example where an API was manufactured has been cleaned up to spec through Swab TOC tests or Surfactant tests. Some validation SOP's test for bacteria on surfaces for example using a Swab test.
Process validation: The collection and evaluation of data, from the process design stage through commercial production, which establishes scientific evidence that a process is capable of consistently delivering quality products.
For example in manufacturing of tablets a final mixing step is validated by withdrawing samples from all points in mixer at intermittent intervals , and assay of active ingredients is done, results are plotted against respective sample points and time intervals , the most efficient time interval at which there are consistent and satisfactory result for desired content at all sampling points is considered to be the best for the process of final mixing step, and this best time interval is again validated by crosschecking and documenting on further three batches. It is one of example of process validation , it can extend to other processes adapted in pharma manufacturing i.e control of the entire processes for repeated batches or runs.
Method Validation is used in labs to prove that a measuring technique is accurate. This involves calibration using standard reagents.
Key players in the pharmaceutical water treatment industry are:
Veolia
GE Water
MECO from the USA for Distillation Stills.
Compiled by Rami E. Kremesti M.Sc., CSci, CEnv, CWEM
Last updated 23-9-2017