
TRANSFORMING BASE SUBSTANCES INTO GOLD

TRANSFORMING BASE SUBSTANCES INTO GOLD
Ozone pre-treatment and post-treatment
Ozone in Water Treament: Ozone is highly reactive gas with a pungent smell composed of three atoms of oxygen . It has a complex impact on water/wastewater parameters – it reduces color, improves taste, odor, kills bacteria, viruses, oxidizes ferrous iron, manganese, cyanide, phenol, benzene, chlorophenol, atrazine, nitrobenzene and other pollutants. Ozone application increases the biodegradability of wastewater and hence COD:BOD ratio decreases.
Ozone Treatment of Return Activated Sludge (RAS) in Sewage Treatment
MLR is an internal recirculation from aerobic to anoxic tanks and RAS is from the secondary sedimentation to incoming flow from primary treatment. The high water content, compressible and colloidal nature of the sludge are common characteristics found in WWTP. Filamentous bacteria are a normal part of the activated sludge microflora while excessive long filaments lead to sludge bulking (floating sludge) and prevent sedimentation. Dosing ozone in the RAS promotes the growth of floc forming bacteria and inhibits the activity of filamentous bacteria which enhances sludge bulking and inhibits sedimentation. Low concentrations of ozone can be used as an approach to promoting floc formation and inhibit the activity of filamentous bacteria and sludge bulking during process.

Figure 1. Floating Bulked Sludge
Ozone disinfection in post-treatment
Disinfection of water using ozone is advantageous compared to more traditional methods, such as chlorine, bromine or UV disinfection. Ozone effectively breaks down the lipid layers in the cell membrane. Ozone is more effective at deactivating viruses and bacteria than any other disinfection treatment, while at the same time requiring very little contact time.
Disinfection of water using ozone has its advantages and disadvantages compared to more traditional methods, such as chlorine or UV disinfection.
Firstly, ozone is more effective at deactivating viruses and bacteria than any other disinfection treatment, while at the same time requiring very little contact time, thus reducing the overall treatment residence time while simultaneously leaving no chemical residues.
Due to the high oxidation potential, ozone will effectively degrade microbes and viruses, causing cell membrane rupture and decomposition of essential biomolecular components in for example bacteria. It works however only in colder waters with a T up to 30 C. At higher temperatures, it flashes too quickly.
There are essentially no harmful residuals from ozone use, as ozone undergoes a natural decomposition in water and reverts back to Oxygen. Ozone treatment also prevents re-growth of micro-organisms, provided that the other processes in the disinfection process have been successful in reducing particulates in the wastewater stream. Ozone is also produced on site and does not require shipping or handling, thus removing complications like safety and environmental issues associated with chemical handling. The fact that Ozone reverts back to Oxygen in water makes the water corrosive to Carbon steel which is one of its disadvantages also. Also any O3 leaks are dangerous to personnel. It is also an expensive technology to implement.
Disinfection efficiency
As already stated above ozonation will enable efficient disinfection. The disinfection efficiency is commonly measured using the CT-value (concentration multiplied by time). In the image below a comparison between ozone and chlorination alternatives is shown.
Ozonation provides protection against essentially all toxic and harmful unwanted microbes. In the table below the CT-values for a range of germs are listed.
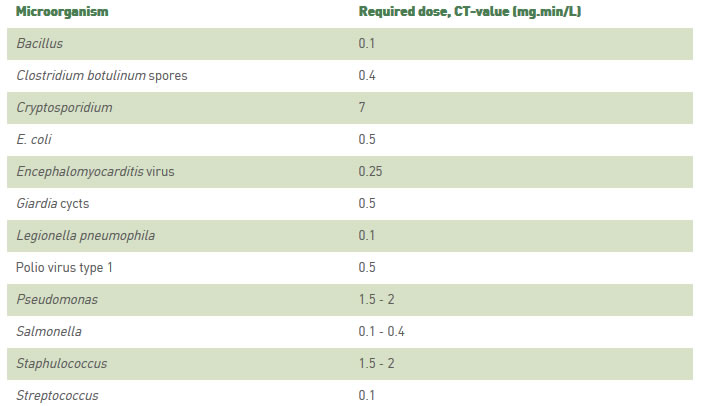
Figure 2. Concentration x Contact Time values for Ozone for various microorganisms
Disinfection prerequisites
Drinking water disinfection is linked to other water purification steps. Proper disinfection can only take place when the water is sufficiently pretreated. In many cases the disinfection process is one of the final steps in a water treament system. In drinking water treatment the disinfection step is preceeded by screening, sedimentation, flocculation, and sand filtration. At this point the water will be suitable for final disinfection.
Dissolved and suspended particles should be removed from water since the particles may contain substrate (food) for pathogenic growth. Moreover, microorganisms are harder to remove from water when they are adsorbed to particles. The concentrations of undissolved particles in water should be reduced below 1 mg/l prior to disinfection. Other chemical compounds from human or natural sources also influence the performance of the disinfection treatment. The substances may react with disinfectants to form disinfection byproducts and increase the Ozone demand to levels that are not economical.
Some Other Applications of Ozone in Water Treatment:
CIP (Clean in Place) of bio-reactors in the Pharmaceutical industry
TOC reduction in demin water production
Bleaching
Bottle Rinsing/Bottled water
Vegetable Washing
Swimming Pools
Cooling Towers
Cooling towers are a relatively new sector for ozone treatment. As such, the benefits of the technology are still being discovered by the users in this sector. The main advantages of ozone water treatment over the traditional chemical water treatment are in the water and energy savings that can be made. The reduction, and possible elimination, of chemical use also creates cost benefits for the user.
The first problem facing water cooling towers is the build up of biological growth and minerals, otherwise known as scale. These problems inhibit the cooling towers’ heat transfer efficiency. The way this problem has been solved in the past has been through the use of chemical agents such as chlorine and chelating/corrosion inhibitor agents. While this serves as an adequate solution to the original problem, the chemicals lead to other problems. Because of the evaporation of water in the tower, the remaining water reaches a high level of chemical and contaminant concentration. To regulate this, water is bled out of the system, and replaced by fresh “make up” water. It is the bleed water that can be problematic to dispose of, with extra sewage cost being incurred.
Ozone treatment solves the original problem with a vastly reduced number of secondary costs and considerations. As well as being a powerful biocide, ozone has been proven to have a positive de-scaling effect. It also greatly reduces the level of bleed off water, as well as the per unit cost of disposing it due to the environmentally friendly nature of ozone. Added to this are the savings due to reduced storage costs and handling of chemicals as ozone is produced on site. This fact significantly simplifies regulatory compliance.
The Ozone mechanism of action
Ozone effectively inactivates and kills microorganisms by oxidizing their organic constituents and rupturing the cell walls. It is a biocidal process to which microbes cannot develop immunity. For example a 0.4 mg/L concentration results in 100 % kill in 2 – 3 minutes for the biofilm producer Pseudomonas fluorescens. A 0.1 mg/L concentration will remove about 80 % of the biofilm in 3 hours. Ozonation technology also has beneficial scaling treatment effects. By removing biofilm to which scale is adhered scaling effects can be significantly reduced when biofilm is present.
Important parameters to consider when using ozone treatment
The following aspects should all be considered when designing, installing, and utilizing ozone in cooling towers:
Practical Ozone Scaling Index (POSI)
To monitor and control scaling when using ozone treatment the POSI index was developed by Pryor and Fischer in 1993. It gives the maximum operation conductivity for the cooling tower to avoid scaling and it takes the reduced amount of dissolved calcium (by using ozonation) into account. The index is explained in the formula below:

Reliable Suppliers of Ozone Generating equipment for Water Treatment Applications:
De Nora, Italy
WEDECO/Xylem
Ozone Tech, Sweden
Prominent Dosiertechnik
References:
Ozone Tech Website https://www.ozonetech.com/
Developed by Rami E. Kremesti M.Sc. CSci, CEnv, CWEM
Contact us for more information on Ozone in Water Treament.