
TRANSFORMING BASE SUBSTANCES INTO GOLD

TRANSFORMING BASE SUBSTANCES INTO GOLD
Septicity in Sewage – Causes and Remedies – Septicity occurs in sewage or sludge when the micro-organisms have utilised all the dissolved oxygen (DO) and any
nitrates (or nitrites) that may be present as an oxygen source for metabolism (electron acceptor). When anaerobic
conditions have developed, proteolytic bacteria in the sewage (or sludge) break down organic sulphur-containing
compounds to sulfides which are smelly. Additionally, sulphate-reducing bacteria utilise sulphates in the water as
an alternative electron acceptor for the dissimilation of organic matter, to form sulphides. Waste water design engineers specify BOD, Ammonia and TP in their designs but forget about Sulfur most of the time…
The consequences of sewage or sludge becoming septic are important for two reasons:
Sulphides produced include hydrogen sulphide and organic sulphides, which have extremely unpleasant odours. If these malodorous sulphides were released into the atmosphere, they can cause a public nuisance.
Additioanlly, Hydrogen sulphide, once released into the atmosphere, can cause corrosion of metals and will also
result in corrosion of concrete on moist surfaces, where it may be biochemically oxidised to sulphuric acid by
various autotrophic thiobacilli bacteria.
ODOURS
Sewage by its nature and composition has an unpleasant odour, which is made obnoxious when it becomes anaerobic and septic. Sludges are more likely to become septic because the numbers of micro-organisms and the available substrates per unit volume are greater. Odours derived from septic sewage or sludge are classified as acidic, which include organic sulphides and hydrogen sulphide (H2S), with odour thresholds within the range 0.5 to 4 ppb, and volatile, organic, fatty-acids, such as acetic, propionic and butyric acid.
If sewage or sludge becomes alkaline, the odours produced, which include ammonia and amines together with other
volatile organic compounds such as skatole and indole, have much higher odour threshold concentrations than acidic
sulphides but are more persistent. Septic sewage does not normally smell of the alkaline-related odours because the
pH value is likely to be less than 7, due to the formation of fatty acids. Measures to control septicity and prevent
odour nuisance must avoid increasing the pH value above about 8.5 or the problem will not be resolved, as malodorous alkaline odours would prevail.
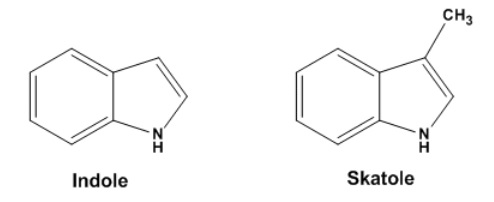
Fig. 1

Fig. 2 – The chemical structure of Butyric Acid (also responsible for the smell of rancid butter)
SEPTICITY – in sewers
Septicity in a sewerage system develops as the result of bacterial activity. Facultative anaerobic bacteria, growing
in sewage and on submerged surfaces, will reduce sulphur-containing compounds and sulphates to form sulphides. This will occur when there is inadequate ventilation and re-aeration of sewage and will most frequently be found where sewage is pumped up completely-filled sewers (rising mains), or in surcharged sewers, out of contact with the atmosphere. The bacteria responsible for sulphide formation are termed, ‘sulphate reducing’ and are in a class which ‘respires’ – sulphate to provide energy for the dissimilation of organic matter. A very small amount of reduced sulphur is assimilated by the bacteria, but most is released into the external environment as sulphides, both inorganic as H2S and organic as a range of sulphides, including methyl sulphide, dimethyl sulphide, methyl disulphide and thiols. All of these sulphides have extremely unpleasant odours at very low concentrations. The process of sulphate ‘respiration’ is analogous to ‘nitrate respiration’, associated with denitrification in an anoxic environment.
Domestic sewage normally contains about 3 to 6 mg/l of organic sulphur, which is present mainly as proteinaceous
matter. In the absence of DO, sulphide will be initially formed by the action of proteolytic bacteria on such
organic sulphur containing compounds, as these bacteria are active at a higher redox potential than those which
subsequently reduce sulphate to form H2S. Sewage contains additional organic sulphur in the form of sulphonates
(about 4 mg/l), derived from household detergents, and inorganic sulphur as sulphates (derived from the potable
water supply) at concentrations that vary depending on water hardness.
The concentration of DO in sewage will depend on turbulence within the sewers and the rate of respiration of the
micro-organisms. In gravity sewers, the slope of the sewer should be adequate to ensure that grit and other debris
do not accumulate. Under normal (average flow-rate) conditions, the velocity of sewage (that is generated by the
slope) should also be adequate to create sufficient turbulence to ensure that the rate at which oxygen dissolves
from the atmosphere in the sewer exceeds the rate of demand by the micro-organisms. Under such conditions, septicity will not occur but volatile and odorous compounds that are always present in sewage will be stripped from solution and into the atmosphere. However, such compounds are unlikely to cause a nuisance, provided that the sewage had not become septic upstream of the turbulent conditions.
If aerobic sewage (containing DO) enters a rising-main sewer, or a slow-flowing gravity sewer, the DO will be used
by the micro-organisms at rates which vary depending on the ‘age’ and temperature of the sewage and the area of the submerged surfaces. The ‘age’ of sewage affects the respiration rate of the sewage because the numbers of micro-organisms will increase the longer that sewage remains in the sewer under aerobic conditions. Eventually, the
respiration rate will reach a maximum as substrates become limited and it will then start to decline. In practice,
the rate of uptake of DO by sewage has been found to vary from 2 to 3 mg/l.h for ‘young’ domestic sewage, up to 14 mg/l.h at 15oC, as the sewage ‘ages’ during its aerobic passage through the sewerage system. The average rate
normally found in the UK is about 6 mg/l.h at 15oC. Bacterial slimes, that adhere to the submerged surfaces and are
generally not limited by available substrate, have been found to consume DO at a rate of about 700 mg/m2/h at 15oC.
The consequence of having these two respiration rates is that the sewage will rapidly become devoid of DO in a
rising main sewer of small diameter, as the surface area per unit volume will be large compared with a sewer of
larger diameter. The rate that micro-organisms consume DO will remain fairly constant until the concentration
reaches about 0.2 to 0.4 mg/l. At about 0.2 mg/l, the rate will decline and asymptote to zero at zero DO. However,
as the DO becomes rate-limiting, any oxidised nitrogen present in the sewage will provide an alternative electron
acceptor for the dissimilation of organic matter. Under such conditions, the micro-organisms will continue to
‘respire’ and oxidise substrate but at a slower rate of about 40 per cent of the aerobic rate.
Under anoxic conditions, the redox potential will decline from about +50 mV to about -100 mV (Eh). When all the
oxidised nitrogen has been utilised, the micro-organisms (proteolytic bacteria) will derive their energy from the
reduction of organic sulphur and sulphates to form sulphides. Sulphate reduction occurs at a redox potential within
the range -200 to -300 mV (Eh), depending on the pH value of the sewage being within the range 6.5 to 8
respectively.
The amount of sulphide formed in a rising-main sewer will be directly proportional to the retention time of the
sewage in the main. The longer the time, the greater the amount formed. Changing the rate or frequency of pumping
will have little or no effect on the amount formed.
The pipe will at all times remain full of sewage and hence the amount formed daily is directly related to the volume
of the sewage in the pipe, the internal surface area of the pipe, the COD (or BOD) and temperature of the sewage and the availability of inorganic and organic sulphur containing compounds. Daily variations in concentration of
sulphide will be directly related to the changes that occur in pumping rates during the day, which will affect the
retention period. Increases in temperature will increase the rate of formation; the rate will double for a 10oC
increase in temperature within the range of about 5 to 25oC.
These processes, which create septicity, also occur when sewage is stored under anaerobic conditions in balancing
and primary settlement tanks.
SEPTICITY IN SLUDGE
The causes of septicity developing in sludges are similar to those given above for sewage in sewers. In sludge, the
numbers of micro-organisms and the availability of substrates will be greater. It is unlikely that settled sludge
(primary, humus or surplus activated) will contain dissolved oxygen. Surplus activated sludge may contain nitrate if
the aerobic treatment process achieves nitrification. Humus sludge from a nitrifying filter is unlikely to contain
nitrate as the retention period in the humus settling tank is likely to be long and any nitrate will have been
‘respired’. Mixtures of primary and secondary sludges will rapidly become anaerobic and then septic, particularly
during warm summer months.
The rate at which septicity develops will depend on the solids content and temperature of the sludge and the
availability of sulphur-containing organic and inorganic compounds. Storage of sludge for more than one to two hours will generally result in sulphides being produced. At the same time, the pH value of the sludge is likely to
decrease as a result of anaerobic fermentation which will produce volatile, organic fatty-acids. Lowering of the pH
value increases the potential for release of sulphides to the atmosphere. Anaerobic digestion of sludge will
continue the process of fermentation and increase septicity, resulting in H2S concentrations in digester gas often
within the range 250 to 1500 ppm (v/v).
EMISSION OF ODOURS
Septicity in sewage and sludge need not create odour nuisance unless the compounds formed are released into the
atmosphere. Volatile organic sulphides formed within sewage or sludge can be oxidised while dissolved. Turbulence of ‘fresh’sewage, will enable oxygen to dissolve from the atmosphere and prevent septicity. Once sewage has become septic, turbulence will release dissolved sulphides into the atmosphere.
Similarly, sludge that has become septic will not create an odour nuisance if the sludge is kept out of contact with
the atmosphere. Avoiding cascades and turbulence will minimise odour release from sludge and into the air.
Minimising retention in holding tanks will reduce the concentration of malodours compounds formed. Retaining sludges in pipes, rather than in ‘open’ channels, and pumping sludge with close-coupled pumps, rather than discharging from bell-mouth pipes, will avoid contact with the atmosphere.
Covering distribution chambers, channels and tanks in which the liquid level remains constant will avoid contact of
sewage and sludge with the air, although the concentrations of malodorous gases below the covers may be very high.
In tanks where the level of liquid varies, providing a cover will not avoid release of malodorous air when the tank
is filling. In such circumstances, the head-space should be sealed and the air ventilated to a suitable treatment
unit, prior to discharge to the atmosphere.
CONTROL OF ODOURS
To prevent septicity of sewage or sludge would require inhibition of the micro-organisms responsible or measures to
prevent anaerobic conditions from developing. Septicity is most serious down-stream from a rising-main sewer, during removal and storage of sludges prior to treatment and disposal and during summer months, when sewage and sludge temperatures are high (20 to 25oC).
In most circumstances, total prevention of septicity would not be practical or economical. Hence, containment to
avoid creating an odour nuisance is likely to be the most effective strategy. Odour problems (and associated
corrosion due to sulphides) could be minimised by:
1. oxidation of H2S before it can be emitted to the atmosphere;
2. conversion of H2S to HS- and S2- by addition of alkali;
3. avoiding turbulent conditions to avoid excessive loss to the atmosphere;
4. scrubbing of vented gases (including air) to remove malodours;
5. avoiding excessive accumulation of debris and grit in pipes and tanks;
6. avoiding unnecessary contact of sewage and sludge with the atmosphere and
7. minimising retention under anaerobic conditions.
Prevention of septicity in sewage and sludges is possible by the use of chemicals:
1. injection of oxygen is used successfully to maintain aerobic conditions in rising-main sewers.
2. addition of nitrate to provide an alternative source of energy for respiration is being used widely to keep
sewage and sludges anoxic.
3. addition of oxidant chemicals, such as hypochlorite, hydrogen peroxide and potassium permanganate is used to
reduce microbial activity and oxidise previously formed sulphides.
4. iron salts added to sewage or sludge will react with sulphides to form insoluble iron sulphide and will catalyse
the rate of oxidation of sulphide. The amount required will depend on the pH value of the sewage or sludge; the dose
rate will be high (about four times the stoichiometric amount) if the pH value is below 6.5.
addition of alkali may be unsuccessful at preventing odour nuisance as it would result in the formation of alkaline
odours.
References
Boon, AG (1995) Septicity in Sewers: Causes, Consequences and Containment. Wat. Sci. Tech. Vol. 31, No. 7, pp 237-
253.
Boon, AG, Vincent, AJ and Boon, KG. (1998) Avoiding the Problems of Septic Sewage. Wat. Sci. Tech. Vol. 37, No. 1,
pp 223-231.
Contact us for more information on Septicity in Sewage – Causes and Remedies