
TRANSFORMING BASE SUBSTANCES INTO GOLD
The Science of Water Treatment Chemicals
by Rami E. Kremesti M.Sc., CSci, CEnv, CWEM
Introduction
The ancients knew that adding silver coins to water jugs helped keep the water fresh. They also used charcoal to help purify water.
Old, stale urine was used to wash wool because urea hydrolysed into ammonia which as a basic substance can help clean off fatty dirt. There is a reference in the Bible to the Fuller’s Field outside Jerusalem. It had to be outside because the place stank. Since then, water treatment chemistry has evolved into an accurate science. There are many chemicals that we use in modern water treatment processes without which, we would have no access to fresh, safe water. Below is a list of some of the major chemicals used in water treatment and their basic chemistry.
Scale and Corrosion Inhibitors:
Usually some kind of blend of phosphonates, phosphates, polymers and azoles, or Silicate, Sodium molybdate, Zinc based corrosion and scale inhibitors, or Aliphatic amine corrosion inhibitors which are sometimes film-forming chemicals. Nitrites are also used as oxidizers for Iron based metallurgy which create a uniform Iron oxide protective film. Please note that while Silicates are useful as scale inhibitors for steel water pipes they would have the opposite effect in RO systems where silica fouls the membranes. For RO systems, calculations are made based on water chemistry and the species that is at risk of forming scale is known and based on this the proper anti-scalant is used.
Flocculants:
Sometimes flocculants such as polyacrylate polymer formulations are dosed before sand filters to aid in the agglomeration process of suspended solids and to improve the efficiency of the filtration process. Flocculants are also dosed in clarifiers after coagulation to aid in the settling process of small suspended solids.
Biocides
can be divided into 2 main groups; oxidising biocides and non-oxidising biocides:
Oxidizing Biocides:
Chlorine or Bromine, Peroxide (ex. Peracetic acid) or Ozone Based. Sodium Per Carbonate is a solid source of hydrogen peroxide and sodium carbonate. Potassium Mono Per-Sulphate KMPS.
Non-Oxidizing Biocides:
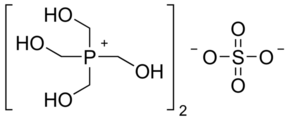
THPS: Tetrakis hydroxymethyl-phosphonium sulfate
ITA: Iso-ThiAzoline
BNP: 2-Bromo-2-Nitro Propan-1,3-diol

Glutaraldehyde:

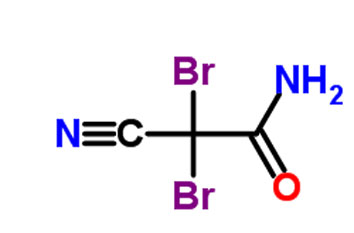
DBNPA: 2,2-dibromo-3-nitrilopropionamide is a quick-kill biocide that easily hydrolyzes under both acidic and alkaline conditions. It is preferred for its instability in water as it quickly kills and then quickly degrades to form a number of products, depending on the conditions, including ammonia, bromide ions, dibromoacetonitrile, and dibromoacetic acid.
Quaternary ammonium chlorides:
Quaternary ammonium salts are used as disinfectants, surfactants, fabric softeners, and as antistatic agents (e.g. in shampoos). In liquid fabric softeners, the chloride salts are often used. In dryer anti-cling strips, the sulfate salts are often used. Spermicidal jellies also contain quaternary ammonium salts.
Polyquats are a variety of engineered polymer forms which provide multiple quat molecules within a larger molecule.
Benzalkonium chloride (above) is a common type of quat salt used as a biocide, a cationic surfactant, and as a phase transfer agent.
Bio Dispersants
do not kill organisms but they do break up any deposits of microorganisms allowing the biocides to attack the organisms more effectively. They also inhibit the attachment of microorganisms to metal surfaces.
Antifoams:
Mineral Hydrocarbons or Silicone based or Mineral oil based antifoams. Note that Mineral based oils are the light distillate fraction of Petroleum oil which contains mostly alkanes.
pH Adjusting Chemicals
If you need the pH to go up, use a base like NaOH or MgOH2 or CaOH2 or Na2CO3. If you want the pH to go down, you use an acid like H2SO4, HCl or NaHSO4 which is a solid and more safe to handle. Optional weaker acids are citric, and acetic acid which are organic.
Descalers:
Sulfamic acid, stabilized HCl, Citric Acid, Phosphoric acid, Formic acid, Acetic acid.
Emulsion Breakers
Demulsifiers, or emulsion breakers, are a class of specialty chemicals used to separate emulsions, for example, water in oil. They are commonly used in the processing of crude oil, which is typically produced along with significant quantities of saline water. This water (and salt) must be removed from the crude oil prior to refining. If the majority of the water and salt are not removed, significant corrosion problems can occur in the refining process.
Demulsifiers are typically based on the following chemistry:
Acid catalysed phenol-formaldehyde resins
Base catalysed phenol-formaldehyde resins
Epoxy resins
Polyethyleneimines
Polyamines
Di-epoxides
Polyols
dendrimer
Cold Lime Softening and Precipitating Chemicals
When water is too hard for some applications, the cold lime softening process is used to remove the hardness from the water. Basically Lime, CaOH2 is added to the water and sometimes Soda Ash to precipitate the Calcium and magnesium in the water. High pH makes calcium precipitate as CaCO3. The same idea is used to remove heavy metals from water and sometimes Sulphides are added which bind strongly to the heavy metals and precipitate.
Chemicals Used in Water Treatment By Application
For Closed Cooling Circuits:
Molybdate / nitrite blend, Phosphate, Nitrite and borate, nitrite based blends are not suitable for Aluminum based systems due to high pH corrosion of Al. Of course some smart chemists know that for all ferric metallurgy, all you need is demin water and a little NaOH to bring the pH up to 10 and you will have no problems with corrosion, scale, bacteria or fouling. The trick is that the system needs to be air tight as Demin water with dissolved oxygen in it is aggressive to steel.
Chemical Free closed cooling water treatment is increasing in popularity. One company that offers this technology is IWTM.
Anti-Freeze:
Mono ethylene glycol, Mono propylene glycol
Chemicals Used In Sewage Treatment
One of the main chemicals used is for control of Phosphorus. Keep in mind that Total Phosphorus can be either organic or inorganic, dissolved or in the form of suspended solids. Chemicals such as Ferric Chloride or Poly Aluminium Chloride are dosed to react with Inorganic Phosphorus and form precipitates. They are also coagulants so they will help lower suspended solids. Note that organic phosphorus can be more difficult to remove.
In sewage treatment plants where the incoming water is low in alkalinity, chemicals such as NaHCO3 or Na2CO3 may be dosed to increase the alkalinity to neutralize the acids generated by septicity or nitrification. Sodium Bicarbonate is a buffer: it can react with acids as well as with bases. Nitrates are sometimes added if there is sulphate in the water to prevent the formation of smelly H2S gas.
Of course, it is know universally that sometimes the effluent from sewage treatment plants is disinfected using Sodium Hypochlorite.
Experiments performed by Kremesti Environmental have proven that adding Magnesium Chloride to raw sewage reduces ammonia in the effluent. This could be owing to the formation of Struvite or the stimulation of nitrifying bacteria which use Magnesium as a nutrient. We published a Case Study about this.
Chemicals Used in Boiler Water Treatment:
There are several categories of chemicals used. Take a look at our presentation on Boiler Water Treatment for a better understanding of the subject.
The main issues are control of pH for increased corrosion resistance using chemicals such as Ammonia and Sodium Phosphate. Then there are the oxygen scavengers.
Lower pressure boilers use dispersants in their steam drums to keep the solids in suspension. Some boilers inject Oxygen gas to form a strong corrosion resistant Hematite layer on the steel.
Drinking Water Treatment:
Drinking water treatment is about producing safe and wholesome water with no smell or taste in it. Surface water sources need to be clarified and filtered. Heavy metals need to be removed if they are in the source water. Bacteria and viruses need to be removed/killed. Some people like their drinking water to be soft and soften it for applications like laundry and dish washing.
Sometimes lead corrosion inhibitors are added to the water to protect the owners of old homes that have lead pipes from lead poisoning. This is normally some kind of phosphate or phosphoric acid. Ocourse we all know that adding a little Sodium Fluoride to drinking water helps with the protection of teeth from cavities.
In reactors that are used to remove heavy metals, sometimes NaOH or CaOH2 are added to precipitate heavy metals. Note that the Ksp curve of the heavy metal or metals in questions need to be studied carefully as some heavy metals precipitate at lower pH ranges and redissolve at higher pH ranges.
Swimming Pools
Pool water treatment uses biocides, algaecides, pH adjusting chemicals as well as coagulants. Read our page on Swimming Pool Water Treatment. Some surface active chemicals are sometimes added to swimming pools in hot water climates which delay evaporation !!!
Anaerobic Digesters
Sometimes AD’s need attention to the water chemistry. Buffers are important as some metabolic processes lower the pH which becomes toxic to the micro-organisms. Also macro and micro nutrients need to be available. ZVI (Zero Valence Iron) or GAC are sometimes added which help as electron donors and ammonia adsorbers respectively. Download our presentation on Anaerobic Digesters.
Chemicals Used for Snow Thawing
Sea salt and Calcium Magnesium Acetate (More environmentally friendly) are used to help prevent the freezing of major highways in winter.
Chemicals Used in Electrowinning
Cetyl-pyridinium chloride is a cool molecule that is used both as a mouthwash anti-septic and at the same time it is used as an inhibitor of the Hydrogen Evolution Reaction in electro-winning processes which aim to recover dissolved metals from solution. Owing to its property of sticking to metal surfaces and its quat ammonium moiety, it is also used in the petroleum industry to protect pipes from corrosion and biofilm formation.
Cetyl- is a prefix that means 16 in organic chemistry and it is derived from cetyl-alcohol which used to be extracted from whales and Cetus means whale in Latin, in Bulgarian it is Kyit.
Struvite Recovery
Magnesium salts like MgO are added to phosphate/ammonia rich sludge dewatering liquor to recover Struvite, or Magnesium Ammonium Phosphate which is an excellent fertilizer. It is interesting to know that phosphorus can be organic or inorganic. Inorganically bound phosphorus is less available to crops as a fertiliser so it is less sustainable to remove P using inorganic precipitatiion.
Global Suppliers of Water Treatment Chemicals
For Oil and Gas Industry
Arkema
Baker Hughes
BASF
ChampionX
Clariant
Dow Chemical Company
Lubrizol
Nouryon
Univar
Buckman Laboratories
Major European suppliers of water treatment chemicals include Ecolab, Kemira, Veolia, Solvay, SNF, Solenis, Kurita, SNF, Brenntag and Feralco.
Chinese suppliers of water treatment chemicals include specialized companies such as Wuxi Lansen Chemicals, Shandong XinTai Water Treatment Technology Co., Ltd., HIXENchem, and IRO Water Treatment, offering products like coagulants, flocculants, scale and corrosion inhibitors, and disinfectants for industrial, municipal, and commercial applications.
Market Size
The global market for water treatment chemicals is estimated to be around USD 37-39 billion in 2024, with projections to reach approximately USD 56-61 billion by 2032, growing at a compound annual growth rate (CAGR) of roughly 4-6%. Key market drivers include growing awareness of water quality, stricter government regulations, and increasing demand from industries such as power generation, oil & gas, and manufacturing.
Contact us for more information on The Science of Water Treatment Chemicals
Biography of the Author:
Rami Elias Kremesti is a chartered water and wastewater treatment specialist with a background in chemistry. He has worked on a myriad of water treatment and power station projects internationally. He is a British Citizen based out of High Wycombe, UK. He has published three books on philosophical topics which he loves to ponder in his spare time.
If you found this page useful, please leave us a positive review on Google.

Rami Elias Kremesti Portrait