
Fertilising The Oceans with Iron to Capture Carbon by Promoting Phytoplankton Growth
by Rami Elias Kremesti M.Sc., CSci, CEnv, CWEM
© 2025 Kremesti Environmental Consutling Ltd
Published with CIWEM click here for link
Introduction
Fertilising the oceans with iron is an emerging idea for fighting rising atmospheric levels in CO2 that cause climate change. I was recently watching Sir David Attenborough’s 2025 movie Ocean where he said something that caught my attention: the oceans’ whales fertilize the water with their faeces which contains iron, a necessary nutrient for phytoplankton, which themselves are the building block of life in the ocean. The idea hit me: what if we fertilise the ocean with Iron? More phytoplankton means more captured atmospheric CO2…
The 2025 UN Ocean Conference, took place in Nice, France, from June 9-13 and addressed various ocean-related issues, including the impact of climate change, pollution, and overexploitation of marine resources. The conference primarily focused on accelerating action and mobilizing all actors to conserve and sustainably use the ocean.
Many parts of the ocean experience iron deficiency, particularly in open ocean areas. While iron is abundant on Earth, it’s less available in the ocean due to reactions with oxygen and formation of poorly soluble minerals. This can limit the growth of phytoplankton, which are the corner stone for the marine food web and carbon cycling. Phytoplankton need Iron for their enzymes. Dust storms natural fertilize the Ocean with Iron. What if we fertilize it artificially to boost the natural phytoplankton cycle and thus capture atmospheric CO2?
John Martin , a famous oceanographer from Connecticut, USA was known for his research on the role of iron as a phytoplankton micronutrient, and its significance for so-called “High-Nutrient, Low Chlorophyll” regions of the oceans. He advocated the use of iron fertilization to enhance oceanic primary production and act as a sink for fossil fuel carbon dioxide.
“Give me a half tanker of iron, and I will give you an ice age.” – John Martin
Ocean pH and Temperature
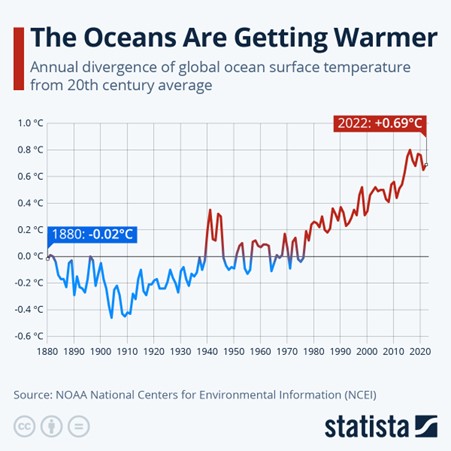
The pH of the oceans is becoming more and more acidic as anthropogenic CO2 levels in the atmosphere continue to rise. Over the past 100 years, the average pH of the oceans has declined from about 8.2 to 8.0 which is stressing the Calcium Carbonate equilibrium that helps crustaceans develop their hard shells. A pH of around 7.7 is often cited as a point where shell formation becomes problematic. At this level, shells may become thinner, growth rates may decrease, and dissolution may occur.

Figure 1: Ocean pH is showing a decreasing alarming rate
The temperature of the ocean is also rising at an alarming rate as a consequence of global warming. Warmer ocean waters can lead to fish death through several mechanisms. Primarily, warmer water holds less dissolved oxygen, leading to deoxygenation and making it harder for fish to breathe. Additionally, warmer temperatures increase fish’s metabolic rates, meaning they need more oxygen to survive, further exacerbating the issue. This can lead to fish “suffocating” in oxygen-poor water. Furthermore, warmer waters can cause shifts in ecosystems, potentially leading to food shortages or the introduction of new predators that local fish are not adapted to, creating stress and even death.
Biochemistry
Iron plays a crucial role in phytoplankton’s growth and photosynthesis, acting as a necessary co-factor for various enzymes. It’s involved in energy production, biochemical catalysis, and the synthesis of chlorophyll. Iron deficiency can limit phytoplankton growth and ocean productivity, impacting the marine food web and carbon sequestration.
Proof of Concept Experiments
Iron fertilization, the practice of adding iron to nutrient-poor regions of the ocean to stimulate phytoplankton growth, is a proposed method for capturing atmospheric CO2. Estimates suggest that iron fertilization could potentially remove 1 gigatonne (1 billion tons) per year of atmospheric CO2. Current annual anthropogenic CO2 emissions are around 37.4 gigatons from fossil fuels and land-use change. This translates to a potential reduction of 2.6 percent of current annual anthropogenic CO2 emissions. Ofcourse this is scalable. More Ocean Iron Fertilization (OIF) leads to more CDR – Carbon Dioxide Removal.
Several experiments, like LOHAFEX (2009), EIFEX in 2004 (The European Iron Fertilization Experiment) and SEEDS in 2001 (The Subarctic Pacific Iron Experiment for Ecosystem Dynamics Study), have successfully demonstrated that introducing iron (often in the form of iron sulfate) to ocean areas can trigger significant phytoplankton blooms. These blooms are visually evident through satellite imagery and measurable through changes in chlorophyll concentrations.
We can add iron to the oceans through several ways: add Ferric salts, install iron rigs that can corrode slowly and release iron into the ocean, or we can accelerate this process through electrolysis: install floating solar powered rigs that generate DC current which can dissolve attached iron electrodes. While natural corrosion takes long periods of time, electro coagulation can produce Ferric ions instantaneously.
Using Electro-Coagulation to produce Ferric in the ocean has a two fold benefit: First it produces ferric through the dissolution of the sacrificial anode and it also produces OH- hydroxide ions at the cathode where Hydrogen gas is produced through the reduction of H+ ions.
The chemical reaction has the added benefit of increasing the alkalinity of the water which can help offset which is a great concern to biologists of marine systems.
Ideas
We can add iron to the oceans through several ways: add Ferric salts, install iron rigs that can corrode slowly and release iron into the ocean, or we can accelerate this process through electrolysis: install floating solar powered rigs that generate DC current which can dissolve attached iron electrodes. There are many voices opposing this practice for various reasons. Some say that Phytoplankton will rob other systems from N and P. Some say that the biomass rots/oxidises thus producing Methane and CO2 instead of sinking. Some say that phytoplankton death results in eutrophication.
The Maths
Iron fertilization, the practice of adding iron to nutrient-poor regions of the ocean to stimulate phytoplankton growth, is a proposed method for capturing CO2. Estimates suggest that iron fertilization could potentially remove 0.1 to 5+ billion metric tons of CO2 per year, with 1 gigatonne (1 billion tons) per year considered most likely. This translates to a potential reduction of 10 to 50 percent of current anthropogenic CO2 emissions.
Carbon Sequestration:
When phytoplankton die, they sink to the ocean depths, carrying the carbon they have absorbed with them. This process, known as the biological pump, can help remove CO2 from the atmosphere and store it in the deep ocean for extended periods. Alternatively, phytoplankton get metabolized by various sea creatures that capture the metabolized CO2 in their own bodies. A dead wale that sinks to the bottom of the oceans buries tons of CO2…
Environmental Concerns:
Conclusion
About the Author

Rami Elias Kremesti Portrait